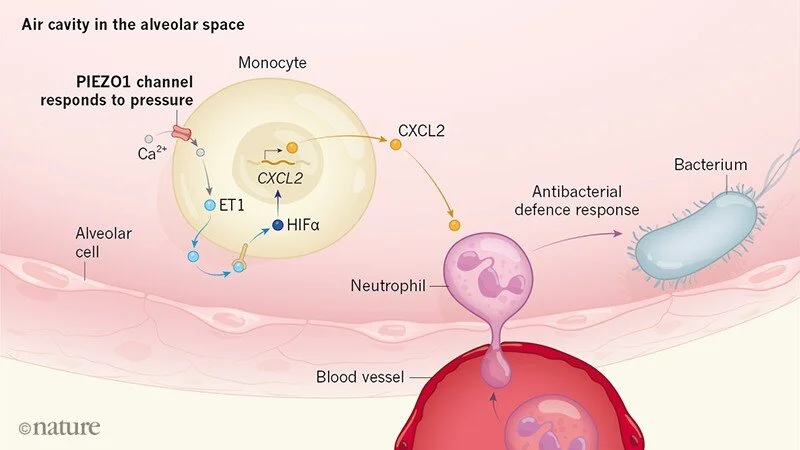
Physical Force Activates the Immune System
Immune cells are subjected to a number of biochemical and physical forces upon recruitment to the site of inflammation. Parameters such as acidity, oxygen availability, and osmolarity are all known to have severe consequence to the mammalian cell. In fact, alterations in these homeostatic conditions are known to regulate the immune response in order to tailor appropriate inflammatory consequences. Physical force is one such environment condition that has been understudied in relation to immunity. Immune cells are subjected to an astounding amount of physical force, and recruitment of immune cells into mechanical organs, such as the heart of lungs, can theoretically affect their antimicrobial response. We characterize the importance of physical force in immunity and identify the mechanosensory ion channel PIEZO1 as a novel trigger for inflammation.
Immune System Disobeys Laws of Genetics
When the human genome was sequenced, researchers had to make decisions to identify protein coding regions. These “canonical open reading frames” typically start with the amino acid methionine and have at least 100 amino acids. But do these parameters capture the full extent of human proteins? Using novel tools, we are able to capture RNA molecules that are actively being translated by the ribosome. From these analyses, we identify novel “non-canonical open reading frames” from immune cells during an active immune response, and characterize their importance in antimicrobial immunity.
Peripheral Nerves in the Intestine Protect Against Bacterial Infection
More and more emphasis is placed on the immunological aspects of traditionally non-immunological cells. For example, we recognize cells of the intestinal epithelia as critical drivers of inflammation. Endothelial cells of the blood vessels can also dictate the immunological response in a number of different ways. But what about nerves? The intestine houses a sizable population of peripheral nerves that are known to play important roles in intestinal motility, but is this all they do? We explore the hypothesis that peripheral nerves are capable of mounting significant immune responses. In our work, we show that specific deletion of inflammatory proteins from these peripheral nerves results in significant consequences during the antibacterial response.